Topic: Structure of Atom, Test No.: 02, Total MCQs: 15
Congratulations - you have completed Topic: Structure of Atom, Test No.: 02, Total MCQs: 15.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
The pair of species with the same bond order is [CBSE
AIPMT 2012]
NO, CO
| |
N2,
O2 | |
O22-,
B2 | |
O2+,
NO+ |
Question 2 |
Bond order of 1.5 is shown by [CBSE AIPMT 2012]
O22- | |
O2 | |
O2+ | |
O2- |
Question 3 |
If the 1st ionization
energy of H atom is 13.6 eV, then the 2nd ionization
energy of He atom is [West Bengla JEE 2012]
27.2 eV | |
40.8 eV | |
54.4 eV | |
108.8 eV |
Question 4 |
The frequency of light emitted for the transition n
= 4 to n = 2 of He+ is equal to the transition in H atom
corresponding to which of the following [AIEEE 2011]
n = 4 to n = 3 | |
n = 3 to n = 1 | |
n = 2 to n = 1 | |
n = 3 to n = 2 |
Question 5 |
The maximum number of electrons that can have principal quantum number, n = 3, and spin quantum number, ms = 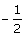 , is [IIT JEE 2011]
, is [IIT JEE 2011]
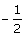 , is [IIT JEE 2011]
, is [IIT JEE 2011]5 | |
7 | |
9 | |
11 |
Question 6 |
The set of quantum numbers for the outermost
electron for copper in its ground state is [Karnataka CET 2010]
3, 2, 2, + 1/2 | |
4, 1, 1, + 1/2 | |
4, 2, 2, + 1/2 | |
4, 0, 0, + 1/2 |
Question 7 |
Calculate the wavelength (in nanometer) associated
with a proton moving at 1.0 × 103 ms−1
(Mass of proton = 1.67 × 10−27 kg and h = 6.63 × 10−34 Js ) [AIEEE 2009]
0.032 nm | |
0.40 nm | |
2.5 nm | |
14.0 nm |
Question 8 |
In an atom, an electron is moving with a speed of
600 m/s with an accuracy of 0.005%. Certainity with which the position of the
electron can be located is (h = 6.6 × 10–34 kg m2s–1,
mass of electron, em = 9.1 × 10–31 kg) [AIEEE 2009]
5.10 ×
10–3 m | |
1.92 ×
10–3 m | |
3.84 ×
10–3 m | |
1.52 ×
10–4 m |
Question 9 |
The ionization enthalpy of hydrogen atom is 1.312 ×
106 J mol−1. The energy required to excite the
electron in the atom from n = 1 to n = 2 is [AIEEE 2008]
8.51 × 105 Jmol−1 | |
6.56 × 105 Jmol−1 | |
7.56 × 105 Jmol−1 | |
9.84 × 105 Jmol−1 |
Question 10 |
When the azimuthal quantum number has the value of
2, the number of orbitals possible are [Karnataka CET 2008]
7 | |
5 | |
3 | |
0 |
Question 11 |
Which of the following sets of quantum numbers
represents the highest energy of an atom? [AIEEE 2007]
n = 3, l = 2,m = 1, s = +1/ 2 | |
n = 3, l = 2,m = 1, s = +1/ 2 | |
n = 4, l = 0,m = 0, s = +1/ 2 | |
n = 3, l = 0,m = 0, s = +1/ 2 |
Question 12 |
A body of mass 10 mg is moving with a velocity of
100 ms-1. The
wavelength of de-Broglie wave associated with it would be
(Note: h = 6.63 × 10-34 Js) [Karnataka CET 2007]
6.63 × 10-37m | |
6.63 × 10-31m | |
6.63 × 10-34m | |
6.63 × 10-35m |
Question 13 |
Uncertainty in the position of an electron (mass =
9.1 × 10–31 kg) moving with a velocity 300 ms–1,
accurate upto 0.001%, will be
(h = 6.63 × 10–34 Js) [AIEEE 2006]
19.2 × 10–2 m | |
5.76 × 10–2 m | |
1.92 × 10–2 m | |
3.84 × 10–2 m |
Question 14 |
In a multi – electron atom, which of the following
orbitals described by the three quantum numbers will have the same energy in
the absence of magnetic acid and electric fields? [AIEEE 2005]
(a) n = 1, l = 0, m = 0
(b) n = 2, l = 0, m = 0
(c) n = 2, l = 1, m = 1
(d) n = 3, l = 2, m = 1
(e) n = 3, l = 2, m = 0
(a) and (b) | |
(b) and (c) | |
(c) and (d) | |
(d) and (e) |
Question 15 |
Which of the following statements in relation to the
hydrogen atom is correct? [AIEEE 205]
3s orbital is lower in energy than 3p orbital | |
3p orbital is lower in energy than 3d orbital | |
3s and 3p orbitals are of lower energy than 3d orbital | |
3s, 3p and 3d orbitals all have the same energy |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 15 questions to complete.

