| Chemistry Questions: Paper - 35 | <<Previous | More Chemistry Questions |

Custom Search
|
Chemistry Model Question Paper - MCQs Test 35 |
||
Sr. No. |
Question |
Answer |
|
1. |
The enthalpy of vaporization
of benzene is +35.3 kJ/mol at its boiling point of [in J mol–1 K–1] (A) +100 (B) –100 (C) –342 (D) +342 |
Answer: (B) |
|
2. |
The formation
of (A) O2 and Xe have comparable sizes (B) both O2 and Xe are gases (C) O2 and Xe have comparable ionisation energies (D) O2 and Xe have comparable electronegativities |
Answer: (C) |
|
3. |
The formula mass of Mohr's salt is 392. The iron present in it is oxidised by KMnO4 in acid medium. The equivalent mass of Mohr's salt is (A) 392 (B) 31.6 (C) 278 (D) 156 |
Answer: (A) |
|
4. |
The geometry of electron pairs around I in IF5 is (A) Octahedral (B) Trigonal bipyramidal (C) Square pyramidal (D) Pentagonal planar |
Answer: (A) |
|
5. |
The half life time of 2g sample of radioactive nuclide 'X' is 15 min. The half life time of 1g sample of X is (A) 7.5 min (B) 15 min (C) 22.5 min (D) 30 min |
Answer: (B) |
|
6. |
The highest magnetic moment is shown by the transition metal ion with the configuration (A) 3d2 (B) 3d5 (C) 3d7 (D) 3d9 |
Answer: (B) |
|
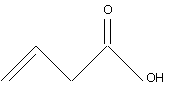
7. |
The IUPAC name of
is (A) but-1-enoic acid (B) but-3-enoic acid (C) prop-2-enoic acid (D) pent-4-enoic acid |
Answer: (B) |
|
8. |
The IUPAC name of the complex [Co (NH3)4Cl2] Cl is (A) tetraammine dichloro cobalt (III) chloride (B) dichloro tetraammine cobalt (III) chloride (C) tetraammine dichloro cobalt (IV) chloride (D) tetraammine dichloro cobalt (II) chloride |
Answer: (A) |
|
9. |
The mass of glucose that should be dissolved in 50 g of water in order to produce the same lowering of vapour pressure as is produced by dissolving 1 g of urea in the same quantity of water is (A) 1 g (B) 3 g (C) 6 g (D) 18 g |
Answer: (B) |
|
10. |
The maximum number of possible isomers in 1-bromo-2-methyl cyclobutane is (A) 2 (B) 4 (C) 16 (D) 8 |
Answer: (B) |
|
11. |
The pH of 10-8 M HCI solution is (A) 8 (B) more than 8 (C) between 6 and 7 (D) slightly more than 7 |
Answer: (C) |
|
12. |
The radii of Na+ and Cl- ions are 95 pm and 181 pm respectively. The edge length of NaCl unit cell is (A) 276 pm (B) 138 pm (C) 552 pm (D) 415 pm |
Answer: (C) |
|
13. |
The reaction C2H5ONa + C2H5I ® C2H5OC2H5 + NaI is known as (A) Kolbe's synthesis (B) Wurtz's synthesis (C) Williamson's synthesis (D) Grignard's synthesis |
Answer: (C) |
|
14. |
The rms velocity of hydrogen
is (A) (B) (C) (D) |
Answer: (D) |
|
15. |
The solubility of Ca3(PO4)2 in water is y moles / litre. Its solubility product is (A) 6y4 (B) 36y4 (C) 64y5 (D) 108y5 |
Answer: (D) |