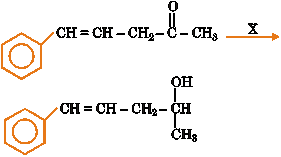| Chemistry Questions: Paper - 32 | <<Previous | More Chemistry Questions |

Custom Search
|
Chemistry Model Question Paper - MCQs Test 32 |
||
Sr. No. |
Question |
Answer |
|
1. |
In the conversion of
X is (A) H2/Pt (B) Zn-Hg/HCl (C) Li/NH3 (D) NaBH4 |
Answer: (D) |
|
2. |
In the conversion
of Br2 to (A) zero to +5 (B) +1 to +5 (C) zero to -3 (D) +2 to +5 |
Answer: (A) |
|
3. |
In the following electron – dot structure, calculate the formal charge from left to right nitrogen atom;
(A) –1, –1, +1 (B) –1, +1, –1 (C) +1, –1, –1 (D) +1, –1, +1 |
Answer: (B) |
|
4. |
In the following, the element with the highest ionisation energy is (A) [Ne]3s23p1 (B) [Ne]3s23p3 (C) [Ne]3s23p2 (D) [Ne]3s23p4 |
Answer: (B) |
|
5. |
In the reaction R - X the product B is (A) alkyl chloride (B) aldehyde (C) carboxylic acid (D) ketone |
Answer: (C) |
|
6. |
In the reaction 2H2O2 ® 2H2O + O2 (A) Oxygen is oxidised only (B) Oxygen is reduced only (C) Oxygen is neither oxidised nor reduced (D) Oxygen is both oxidised and reduced |
Answer: (D) |
|
7. |
In Tollen's test, aldehydes (A) Are oxidised to acids (B) Are reduced to alcohol (C) Neither reduced nor oxidised (D) Precipitate Ag+ as AgCI |
Answer: (A) |
|
8. |
In which of the following complex ion, the central metal ion is in a state of sp3d2 hybridisation? (A) [CoF6]3- (B) [Co(NH3)6]3+ (C) [Fe(CN)6]3- (D) [Cr (NH3)6]3+ |
Answer: (A) |
|
9. |
In which one of the given formulae of xenon compounds there are five a-bonds and three p-bonds in it? (A) XeFO (B) XeF2O2 (C) XeF3O2 (D) XeF2O3 |
Answer: (D) |
|
10. |
Increasing order of carbon-carbon bond length for the following is C2H4 C2H2 C6H6 C2H6 (A) (B) (C) (D) (A) C < B < A < D (B) B < C < A < D (C) D < C < A < B (D) B < A < C < D |
Answer: (D) |
|
11. |
Inductive effect involves (A) displacement of s electrons (B) delocalisation of p electrons (C) delocalisation of s electrons (D) displacement of p electrons |
Answer: (A) |
|
12. |
Insulin regulates the metabolism of (A) minerals (B) amino acids (C) glucose (D) vitamins |
Answer: (C) |
|
13. |
Liquor ammonia bottles are opened only after cooling. This is because (A) it is a mild explosive (B) it is a corrosive liquid (C) it is a lachrymatory (D) it generates high vapour pressure |
Answer: (D) |
|
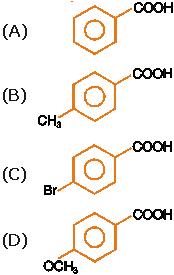
14. |
Lowest pKa is associated with
|
Answer: (C) |
|
15. |
Maximum efficiency of a commercial refrigerator which operates between –10° (inside temperature) and 25°C (outside temperature) is (A) 13.3% (B) 11.45% (C) 24.75% (D) 20% |
Answer: (B) |