Topic: d and f Block Elements, Test No.: 04, Total MCQs: 10
Congratulations - you have completed Topic: d and f Block Elements, Test No.: 04, Total MCQs: 10.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
The outer
electron configuration of Gd (Atomic number: 64) is:
4f3
5d5 6s2 | |
4f8
5d0 6s2 | |
4f4 5d4
6s2 | |
4f7
5d1 6s2 |
Question 2 |
The oxidation
number of Mn in the product of alkaline oxidative fusion of MnO2 is
2 | |
3 | |
4 | |
6 |
Question 3 |
The ground
state electronic configuration of CO molecule is
1s22s21p43s2 | |
1s22s23s21p22p2 | |
1s22s21p23s22p2 | |
1s21p42s23s2 |
Question 4 |
The
increasing order of the ionic radii of the given isoelectronic species is:
Cl–,
Ca2+, K+, S2– | |
S2– ,
Cl–, Ca2+, K+ | |
Ca2+,
K+ , Cl– , S2– | |
K+, S2–,
Ca2+, Cl– |
Question 5 |
Mercury is a
liquid metal because
it has a completely filled s-orbital | |
it has a small atomic size | |
it has a completely filled d-orbital that prevents d-d overlapping of orbitals | |
it has a completely filled d-orbital that causes d-d overlapping |
Question 6 |
Of the
following outer electronic configurations of atoms, the highest oxidation state
is achieved by which one of them?
(n – 1) d8 ns2 | |
(n – 1) d5 ns1 | |
(n – 1) d3 ns2 | |
(n – 1) d5 ns2 |
Question 7 |
The actinoids
exhibits more number of oxidation states in general than the lanthanoids. This
is because
the 5f orbitals are more buried than the 4f orbitals | |
there is a similarity between 4f and 5f orbitals in their angular part of the wave function | |
the actinoids are more reactive than the lanthanoids | |
the 5f orbitals extend further from the nucleus than the 4f orbitals |
Question 8 |
The bonds
present in the structure of dichromate ion are
four equivalent
Cr – O bonds only | |
six equivalent
Cr – O bonds and one O – O bond | |
six equivalent
Cr – O bonds and one Cr – Cr bond | |
six equivalent
Cr – O bonds and one Cr – O – Cr bond |
Question 9 |
The correct
order of 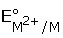 values with negative
sign for the four successive elements Cr, Mn, Fe and Co is
values with negative
sign for the four successive elements Cr, Mn, Fe and Co is
Cr > Mn > Fe > Co | |
Mn > Cr > Fe > Co | |
Cr > Fe > Mn > Co | |
Fe > Mn > Cr > Co |
Question 10 |
The methods
chiefly used for the extraction of lead and tin from their ores are
respectively
self reduction and carbon reduction | |
self reduction and electrolytic reduction | |
carbon reduction and self reduction | |
cyanide process and carbon reduction |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 10 questions to complete.

