Topic: Structure of Atom, Test No.: 08, Total MCQs: 10
Congratulations - you have completed Topic: Structure of Atom, Test No.: 08, Total MCQs: 10.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
The value of
Planck's constant is 6.63 × 10–34 Js. The speed of light is 3 × 1017
nm s–1. Which value is closest to the wavelength in nanometer of a
quantum of light with frequency of 6 × 1015 s–1? [NEET
2013]
25 | |
50 | |
75 | |
10 |
Question 2 |
The values of
four quantum numbers of valence electron of an element are
n = 4, l = 0, m = 0 and s = ![]() . The
element is
. The
element is
K | |
Ti | |
Na | |
Sc |
Question 3 |
The
velocities of two particles A and B are 0.05 and 0.02 ms–1 respectively.
The mass of B is five times the mass of A. The ratio of their de-Broglie's
wavelength
2:1 | |
1:4 | |
1:1 | |
4:1 |
Question 4 |
The
wavelength (in Å) of an emission line obtained for Li2+ during
electronic transition from n2 = 2 to n1 = 1 is (R =
Rydberg constant)
4/3R | |
27R/4 | |
3R/4 | |
4/27R |
Question 5 |
The
wavelength associated with a golf ball weighing 200 g and moving at a speed of
5 m/hour is of the order of:
10–10 m | |
10–20 m | |
10–30 m | |
10–40 m |
Question 6 |
The
wavelength of a spectral line emitted by hydrogen atom in the Lyman series is 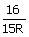 cm.
What is the value of n2 (R = Rydberg constant)
cm.
What is the value of n2 (R = Rydberg constant)
2 | |
3 | |
4 | |
1 |
Question 7 |
The wavelengths of electron waves in two orbits is 3 : 5. The
ratio of kinetic energy of electron will be
25 : 9 | |
5 : 3 | |
9 : 25 | |
3 : 5 |
Question 8 |
What
is the correct order of spin only magnetic moment (in BM) of Mn+2
and V+2 is
Mn2+
> V 2+ > Cr2+ | |
V 2+
> Cr2+ > Mn2+ | |
Mn2+
> Cr2+ > V 2+ | |
Cr2+
> V 2+ > Mn2+ |
Question 9 |
The
H-spectrum show
Heisenberg's uncertainty principle | |
diffraction | |
polarisation | |
presence of quantised energy level |
Question 10 |
The maximum
number of sub-levels, orbitals and electrons is 'N' shell of an atom are
respectively
4, 12, 32 | |
4, 16, 30 | |
4, 16, 32 | |
4, 32, 64 |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 10 questions to complete.

