Topic: Hydrocarbons, Test No.: 02, Total MCQs: 15
Congratulations - you have completed Topic: Hydrocarbons, Test No.: 02, Total MCQs: 15.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Question 1 |
CH3CH2CH2C≡CCH2CH2CH3 | |
CH3CH2C≡CH | |
CH3CH=CHCH3 | |
CH3CH2C≡CCH2CH3 |
Question 2 |
The treatment of CH3MgX with CH3C ≡ C−H produces [AIEEE 2008]
CH3−CH = CH2 | |
CH3C ≡ C−CH3 | |
| |
CH4 |
Question 3 |
Benzene reacts with chlorine in sunlight to give a final product [Karnataka CET 2007]
C6H5Cl | |
C6Cl6 | |
C6H6Cl6 | |
CCl4 |
Question 4 |
The general formula of a cycloalkane is [Karnataka CET 2007]
CnH2n+2 | |
CnH2n–2 | |
CnH2n | |
CnHn |
Question 5 |
Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound E on further treatment with aqueous KOH yields compound F. Compound F is [IIT JEE 2007]

Option (A) | |
Option (B) | |
Option (C) | |
Option (D) |
Question 6 |
The reagent(s) for the following conversion,
![]()
is/are [IIT JEE 2007]
alcoholic KOH | |
alcoholic KOH followed by NaNH2 | |
aqueous KOH followed by NaNH2 | |
Zn/CH3OH |
Question 7 |
activates the ring towards electrophilic substitution | |
renders the ring basic | |
deactivates the ring towards nucleophilic substitution | |
deactivates the ring towards electrophilic substitution |
Question 8 |
The compound formed as a result of oxidation of ethyl benzene by KMnO4 is [AIEEE 2007]
benzophenone | |
acetophenone | |
benzoic acid | |
benzyl alcohol |
Question 9 |
Which of the following reactions will yield 2, 2-dibromopropane? [AIEEE 2007]
CH3 − C ≡ CH + 2HBr → | |
CH3 CH ≡ CHBr + HBr→ | |
CH ≡ CH + 2HBr→ | |
CH3 − CH = CH2 + HBr → |
Question 10 |
HBr reacts with CH2 = CH – OCH3 under anhydrous conditions at room temperature to give [AIEEE 2006]
CH3CHO and CH3Br | |
BrCH2CHO and CH3OH | |
BrCH2 – CH2 – OCH3 | |
H3C – CHBr – OCH3 |
Question 11 |
a mixture of anisole and Mg(OH)Br | |
a mixture of benzene and Mg(OMe)Br | |
a mixture of toluene and Mg(OH)Br | |
a mixture of phenol and Mg(Me)Br |
Question 12 |
A gas decolourised by KMnO4 solution but gives no precipitate with ammoniacal cuprous chloride is [Karnataka CET 2005]
Ethane | |
Methane | |
Ethene | |
Acetylene |
Question 13 |
What would be the product formed when 1-Bromo-3-chlorocyclobutane reacts with two equivalents of metallic sodium in ether? [IIT JEE 2005]
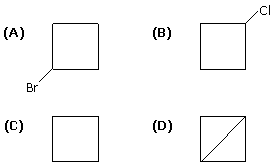
Option (A) | |
Option (B) | |
Option (C) | |
Option (D) |
Question 14 |
1-bromo 2-methylbutane | |
2-bromo 2-methylbutane | |
2-bromo 3-methylbutane | |
1-bromo 3-methylbutane |
Question 15 |
alkanes | |
alkyl copper halides | |
alkenes | |
alkenyl halides |


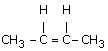
Can u pls explain me ques no 5???
Can I plz explain ques 3 … benzene form only one monosubstituted product in substitution end and it form hexachorobenzene with excess of chlorine in anhydrous medium
Sorry it’s can anyone plz explain
when benzene react with 3 mole of cl2 with sunlight it gives BHC.it is a powerful pesticide.
Usually Benzene doesn’t react with anything in general but since we provide sunlight(which is like drastic condition) the bonds cleave(the pi bonds not sigma), so this results in like a normal cyclo alkane. Then the chlorine reacts(since its excess) with cyclo hexane and forms gammaxene.
when chlorine react with benzene in the presence of sunlight then it break its all double bonds and in place form bonds with chlorine to form the C6H6Cl6